Understanding the Percent Error Formula
The percent error formula is a critical concept in fields like chemistry, physics, engineering, and mathematics. It is used to express the difference between an experimental value and a true or accepted value in percentage terms. This formula helps quantify the accuracy of experimental results and assess the reliability of measurements.
What is Percent Error?
Percent error is a measure of how inaccurate a measurement is, compared to the true value or accepted standard. It is commonly used to assess the precision of an experiment or to determine how close a measured value is to the actual value.
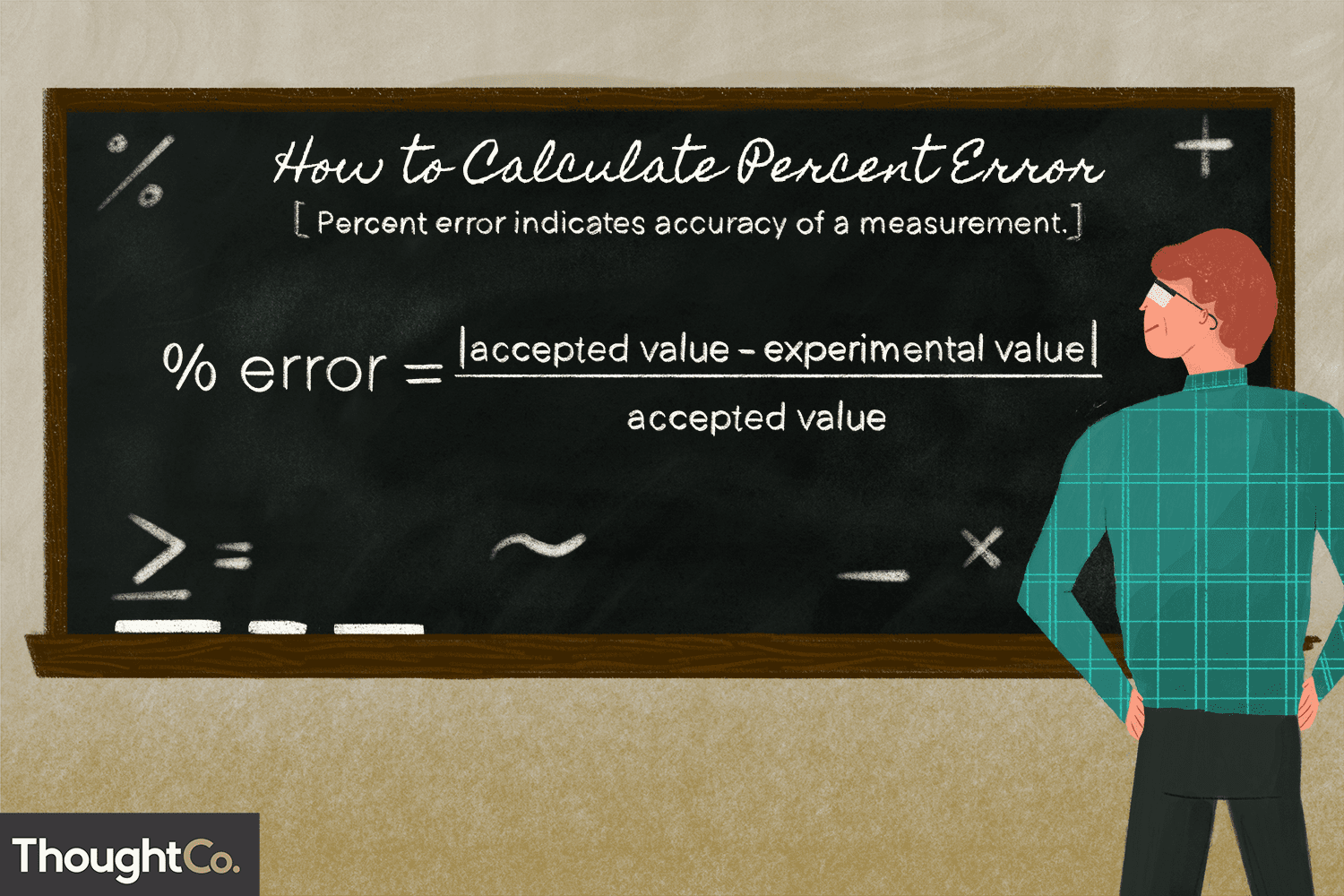
The Percent Error Formula
The percent error formula is given by:
Percent Error=(∣Experimental Value−True Value∣True Value)×100%\text{Percent Error} = \left( \frac{|\text{Experimental Value} – \text{True Value}|}{\text{True Value}} \right) \times 100\%
Here’s a breakdown of the formula:
- Experimental Value: This is the value that you obtain from your experiment or measurement.
- True Value: This is the actual or accepted value, which is considered to be accurate.
- Absolute Value: The absolute value (denoted by the vertical bars) ensures that the result is always positive, reflecting the magnitude of the error regardless of its direction.
Importance of Percent Error
Percent error is essential because it provides a standardized way to compare the accuracy of different measurements. It allows scientists and researchers to determine how close their experimental values are to the true values and to identify potential sources of error in their experiments.
How to Calculate Percent Error
- Determine the Experimental and True Values: Identify the values you obtained from your experiment and the accepted or true value.
- Calculate the Difference: Subtract the true value from the experimental value.
- Take the Absolute Value: Ensure the difference is positive by taking the absolute value.
- Divide by the True Value: Divide the absolute difference by the true value.
- Multiply by 100: Convert the result into a percentage by multiplying by 100.
Example Calculation
Let’s consider an example where you are measuring the density of a substance. Suppose the true density of the substance is 8.96 g/cm³, and your experimental measurement is 9.15 g/cm³.
- Experimental Value: 9.15 g/cm³
- True Value: 8.96 g/cm³
- Difference: 9.15−8.96=0.199.15 – 8.96 = 0.19
- Absolute Value: ∣0.19∣=0.19|0.19| = 0.19
- Divide by True Value: 0.198.96=0.0212\frac{0.19}{8.96} = 0.0212
- Multiply by 100: 0.0212×100=2.12%0.0212 \times 100 = 2.12\%
So, the percent error in this measurement is 2.12%.
Sources of Error
Several factors can contribute to the error in measurements:
- Instrumental Errors: These are errors caused by imperfections in measuring instruments. For example, a scale that is not properly calibrated can give inaccurate readings.
- Human Errors: These are errors caused by human mistakes, such as misreading a measurement or recording incorrect values.
- Environmental Errors: These are errors caused by environmental factors such as temperature, humidity, and pressure that can affect the measurement.
- Methodological Errors: These are errors caused by using incorrect methods or procedures during the experiment.
Reducing Percent Error
To minimize percent error and improve the accuracy of measurements, consider the following tips:
- Calibrate Instruments: Regularly calibrate measuring instruments to ensure they provide accurate readings.
- Repeat Measurements: Perform multiple measurements and take the average to reduce random errors.
- Improve Techniques: Use precise and accurate techniques for measurements to minimize human errors.
- Control Environment: Maintain a controlled environment to minimize the impact of external factors on the measurements.
- Use Proper Methods: Follow standardized methods and procedures to ensure consistency and accuracy.
Conclusion
The percent error formula is a fundamental tool in scientific research and measurement. It helps quantify the accuracy of experimental results and identify potential sources of error. By understanding and applying the percent error formula, scientists and researchers can improve the reliability of their measurements and enhance the overall quality of their experiments. Whether you are conducting a simple classroom experiment or a complex research study, the percent error formula is an invaluable resource for assessing and improving measurement accuracy.